
גלוקוז אמין - האם עוזר לבעיות מפרקים?
Nestlé Purina PetCare January 28, 2003 Product Technology Center
Regulatory Relations
INTERNAL COMMUNICATION
To: C. Cowell
M. Roos
K. Dowling
Cc: D. Chausow
G. Perez-Camargo
From: David Corley
Subject: Literature Review Update on the Oral Use of Glucosamine for Maintaining Healthy Joints and Well-Being in Dogs and Cats
Executive Summary:
A comprehensive review of the literature on nutritional bioactives for osteoarthritis was conducted by Laurence Stoll-Le Guyader (Nestlé Research Center: Project # RE-005900.15, Osteoarthritis and Nutrition, Date: 2002-06-11). This review discussed the use of several dietary nutrients and botanicals in various models of osteoarthritis. A technical communication on the use of glucosamine and chondroitin sulfate in treating osteoarthritic dogs and cats was issued by Fred Xu (Date: 2003-01-10). This present communication is focused on glucosamine, and its natural sources, to support and maintain healthy metabolic functions in the articular joints of dogs and cats.
TABLE OF CONTENTS
1. Introduction........................................................................................... 3
2. Review of Efficacy................................................................................ 4
3. Pharmacokinetics ................................................................................. 6
4.Safety.................................................................................................... 8
5.Mechanism of Action.......................................................................... 10
6. Cartilage as Source of Chondroprotective Agents........................... 11
7. Discussion & Conclusions.................................................................. 12
8. References.......................................................................................... 13
Introduction
Osteoarthritis (OA) is a degenerative joint disease often characterized by chronic and progressive articular cartilage deterioration that is accompanied by clinical signs of pain and loss of mobility. 1-2 There is increasing evidence relating various risk factors such as genetics, diet, bone density, muscle weakness, joint laxity, and obesity to osteoarthritis. A disease with multiple etiologies and risk factors, osteoarthritis is a major contributor to lameness and loss of limb function in dogs and cats.
It has been estimated that as much as 20% of canines over the age of one year suffer from some form of osteoarthritis.2-4 Another study estimated that 3% of all dogs over 10 years of age were diagnosed with osteoarthritis ranking it 4th of the most common diagnosis (Fig. 1). 5 In a recently completed life span study in Labrador Retrievers it was observed that OA was the most prevalent of all the chronic diseases that developed as the dogs aged. 6
-OA is most often diagnosed in older animals, but it can affect young animals as well.
- Signs of OA can be seen and radiographically confirmed in sigle or multiple joints
Figure 1. Top 10 canine diagnoses by category over age 10.
Preliminary data. 1995 National Companion Animal Study. Center for Companion Animal Health, U. of Minnesota, St. Paul and Mark Morris Institute, Topeka, KS.
The incidence of OA in cats (see separate review by D. Corley, 1.27.03) has not been as well documented as it is thought that OA may go unnoticed due to a cat’s small body stature and ability to redistribute its weight to other limbs.7 Certain breeds of cat appear to be prone to hip dysplasia with incidence rates as high as 18-20% using radiographic analysis.8-9 The concern that feline osteoarthritis is under-diagnosed in the veterinary community was highlighted at a roundtable discussion on the geriatric cat.10
Conventional treatment of osteoarthritis is most commonly palliative and includes: weight loss, changes in lifestyle and exercise habits, physical therapy, surgical management, and pharmacological intervention. Drugs that are most often used in OA are nonsteroidal anti-inflammatory drugs (NSAIDS), polysulfated glycosaminoglycans, intra-articular corticosteroids, and IV hyaluronic acid.11
Articular cartilage is composed of chondrocytes that secrete an extracellular matrix comprising proteoglycans, type II collagen, and water that together forms a hydrated gel-like substance.12 Cartilage usually contains no blood vessels so nutrients, hormones, metabolites, and gasses must diffuse through the porous matrix. Cartilage is “shaped” by its surrounding perichondrium, a connective tissue composed of collagen type I and III and proteoglycans.
Like bone, cartilage is in a continual state of remodeling. During growth, cartilage is eroded by osteoclasts to make way for new bone. At maturity, a slight shift in the cartilage anabolic/catabolic equilibrium can lead to a gradual erosion of cartilage over time resulting in osteoarthritis. Degenerative changes develop in the joints of many dogs as they age. More severely affected dogs will show radiographic signs of OA well before the geriatric period, and even as early as one year of age.
A key building block of cartilage is the simple aminosugar glucosamine. Glucosamine, derived from both endogenous biosynthesis and diet, is metabolized into the much larger matrix components of glycosaminoglycans (GAGs) including chondroitin sulfate and hyaluronic acid (Fig. 2). Except for hyaluronic acid, GAGs are found covalently attached to proteins in the form of proteoglycans.
Figure 2. Biosynthesis of glucosamine and GAGs via the Hexosamine Pathway.
Glucose
Glucose-6-phosphate
Fructose-6-phosphate
Glucosamine-6-phosphate
N-Acetylglucosamine
N-Acetylgalactosamine
Chondroitin
Chondroitin sulfate
Glutamine
Glucuronate
Hyaluronic acid
GFAT
UDP-GlcNAc
Glycosylations
Glycogen
98%
1-3%
Dietary Glucosamine
Over the last three decades, an increasing body of evidence supports the use of glucosamine for osteoarthritis. Data from peer-reviewed journalson the effects of oral supplementation with glucosamine, or natural sources of glucosamine, are summarized below.
Review of Efficacy
Chondroprotective agents (CPAs) have an advantage over conventional NSAID treatment for chronic use in osteoarthritis. Although NSAIDs may provide immediate pain relief, their side-effect profile often limits their use to acute treatments. The use of chondroprotective agents spans several decades and is primarily focused on various forms of the chemical components of cartilage including: glycosaminoglycans (GAGs), chondroitin sulfate (CS), glucosamine (GL), and associated vitamins and minerals. Collectively, CPAs have been systematically tested in several species and several models of OA. A key advantage of CPAs are their ability to be administered chronically so that their beneficial effects can be realized.
There have been several clinical trials testing the efficacy and tolerability of glucosamine as a stand-alone treatment or as a combination treatment with chondroitin sulfate (Table 1). Since glucosamine appears to be well tolerated, even a mild positive effect on osteoarthritis may be clinically beneficial.
Table 1. Summary of clinical trials using glucosamine for osteoarthritis
Pharmacokinetics
The bioavailability and pharmacokinetics of glucosamine in rats, dogs and man have been reviewed and clearly indicate that glucosamine is bioavailable and is incorporated into cartilage.23-25 In a recent study using normal beagle dogs, a single dose administered PO showed a Tmax of 1.5 hour and a mean bioavailability of 12% (Table 2). 23
Table 2. Pharmacokinetic parameters for glucosamine hydrochloride.
Using 14C-labeled glucosamine, it was shown that the absorption of glucosamine is relatively fast and the majority is excreted in the urine (Table 3). 24 It was shown in rats, dogs and humans that plasma concentrations of glucosamine freely diffused through a dialyzing membrane, an indication that plasma protein binding is minimal.24 In a study of tissue/organ distribution, glucosamine rapidly diffused to most tissues and organs with a similar distribution profile between rats, dogs, and humans (Table 4). 24-25
Table 3. Cumulated radioactivity found in urine and feces after oral administration to beagle dogs expressed as percent of amount administered ±SD.
Table 4. Organ and tissue distribution of radiolabeled glucosamine after oral administration.
Earlier studies investigating the efficacy and tolerance of glucosamine in humans have reported that glucosamine is well tolerated when administered at an oral dose of 1,500 mg daily for 8 weeks with similar side effects reported between placebo and treated groups.26 In studies comparing the efficacy and tolerance of glucosamine with the anti-inflammatory drugs acetylsalicylic acid and indomethacin, it was concluded that glucosamine had a lower potency, but a significantly higher (10-30x) therapeutic index. This was due to glucosamine having little to no GI toxicity. 27-28
A study to investigate the hematologic and hemostatic effects of chondroprotective agents in normal beagle dogs showed minor, but not clinically important, changes (Table 5).29
Table 5. Median values (min to max) for hematologic data in 10 beagle dogs prior (PRE) to oral administration of chondroprotectant agent and at 3,14, and 30 days of treatment.
Glucosamine is a 6-membered sugar and it was hypothesized that oral administration may induce insulin resistance via a flux of the hexosamine biosynthetic pathway. 30 The “glucosamine hypothesis” is based on the observation that a small amount (1-3%) of the glucose metabolized by insulin-sensitive tissues is through the glucosamine pathway and that an increase flux of this pathway facilitates the desensitization of the glucose transport system and glycogen synthesis (Fig. 2). 31-34 A study testing this hypothesis using an insulin clamp model in rats showed that a prolonged infusion of glucosamineinduced insulin resistance in skeletal muscle in normoglycemic but not hyperglycemic rats.30
Another study testing the same hypothesis showed that short-term infusions of glucosamine in humans had no effect on insulin sensitivity. 35
The enzyme glutamine fructose-6-phosphate amidotransferase (GFAT) is the critical enzyme regulating the flux through the hexosamine pathway. The exact nature of signal translocation of GFAT remains unknown, but the downstream effect is an impairment of insulin-induced GLUT4 translocation, glycogen synthesis, both of which may be secondary to inhibition of insulin signaling by depleting ATP pools. 36-41
It has been proposed that the hexosamine pathway serves as a “fuel sensor” in insulin-sensitive cells. 31,42-44 Acceleration of the hexosamine pathway in vivo can be achieved with glucosamine, uridine, high free fatty acid and glucose levels, and GLUT1 and GFAT over-expression.45 To overcome possible side effects of treating cells with high concentrations of hexosamines, a transgenic cell line over-expressing GFAT was shown to become insulin resistant and the defect in insulin signaling appeared to occur post insulin receptor.43
In a human study designed to prove if glucosamine can diminish the b-cell response to glucose in vivo, no alterations in b-cell secretory patterns were observed during either the low dose or high dose study. This observation was contradictory to previous reports in rats perhaps due to inter-species variations. 46-48 In addition, it was observed in the human study that glucosamine shifted the b-cell glucose threshold to the right, which is consistent with glucosamine acting as a competitive inhibitor of glucokinase. 45
It is now recognized that infusions of glucosamine at high loads can produce insulin resistance in normoglycemic rats. A study was designed to test if oral ingestion of glucosamine at doses relevant to therapeutic doses also produced insulin resistance in a SHR(?) and SD(?) rat model.43 As elevations of systolic blood pressure (SPB) is an early and highly sensitive sign of insulin resistance in these models, SPB was used as the primary endpoint. Each of the four groups for each model was fed either a baseline diet, baseline diet plus 0.5% w/w of glucosamine, baseline diet plus 0.4% chondroitin sulfate, or baseline diet plus glucosamine and chondroitin sulfate at equivalent concentration to the test group. These concentrations represent 10-20 times the human dose of 1,500 mg/day for a 70 kg human (equivalent to 3-7 times human dose for a metabolically adjusted rat). The results after 9 weeks showed that glucosamine alone, and in combination with chondroitin sulfate significantly lowered SBP, the opposite effect of glucose-induced insulin resistance. Also, there was no difference between glucose values after a glucose tolerance test at the end of the 9-week study.
Mechanism of Action
It was observed and documented as early as 1956 that hexosamine salts could stimulate certain pathways associated with cartilage synthesis. 50 In 1963, it was shown that cartilage extracts could stimulate hyaluronic acid release in cultured murine fibroblasts and a follow-up study demonstrated the selectivity of certain hexosamine salts in stimulating mucopolysaccharide (MPS) production (Table 6).51 As shown in Table 6, glucosamine, regardless of salt form, and N-Acetyl-D-galactosamine stimulated MPS production, while other related compounds showed either no effect or a decrease in MPS production. The authors of this study comment that as neither the cell number, nor cell size, changed, the increase in MPS production is due to an increase in MPS biosynthesis of the individual cell. Although the mechanism is unknown, the authors postulate that an additional non-glucose limited pathway might be up-regulated by the addition of glucosamine.51
Table 6. Effects of hexosamine derivatives on mucopolysaccharide (MPS) production in fibroblast monolayer cultures.
Through the 1990s to present, several studies (below) investigated the mechanisms of action of glucosamine and chondroitin sulfate utilizing more advanced tissue culture and biochemical analyses:
-
A 1992 study investigating the effects of glucosamine and other chondroprotective agents on human chondrocytes showed glucosamine having a stimulatory effect on proteoglycans production and no effect on collagen-II or basal PGE2 production. 52
-
A 1998 study on aggrecan (the highly negatively charged aggregating proteoglycan of cartilage) showed that glucosamine could inhibit the aggrecanase (a matrix metalloprotease) response in chondrocytes. As this inhibition was seen in both IL-1a, and retinoic acid stimulated chondrocytes, the authors conclude that the site of glucosamine action is downfield from nuclear signaling events.53
-
A 1999 study demonstrated that exposing bovine cartilage to serum collected from beagle dogs dosed with Cosequin® could both stimulate cartilage metabolism and inhibit cartilage degradation. 54
· A 2000 study showed the ability of glucosamine sulfate to dose-dependently inhibit phosholipase A2 (PLA2) and had a modest inhibitory effect on collagenase activity in cultured human osteoarthritic chondrocytes. Further, this study demonstrated that glucosamine sulfate upregulated protein synthesis and protein kinase-C (PKC) production.55
-
A 2001 study demonstrated a profound effect of glucosamine and N-acetylglucosamine to inhibit IL-1b-mediated activation of NO, COX-2, and IL-6 production in human chondrocytes. These effects were specific to glucosamine and N-acetylglucosamine as other sugars including glucose, glucuronic acid, and N-acetylmannosamine did not have this activity. 56
-
In a study published in 2002, glucosamine was shown to inhibit IL-1 stimulated cartilage degradation in equine cartilage explants. In this study glucosamine inhibited cartilage catabolism in a dose dependent manner for stromelysin activity and collagenase/gelatinase activity. 57
-
Another study published in 2002 investigated the effects of glucosamine on various functions of neutrophils. The authors report that glucosamine dose-dependently affected : superoxide generation, phagocytosis, granule enzyme release, chemotaxis, CD11b expression, actin polymerization and p38 MAPK phosphorylation.58
Cartilage as Source of Chondroprotective Agents
Cartilage has been a constituent of canine and feline ancestral diets long before domestication and remains in the diet today as a component of many commercial feeds. Bovine and poultry remain the most abundant sources of cartilage in modern feeds, though other animal species are used in meal by-products.
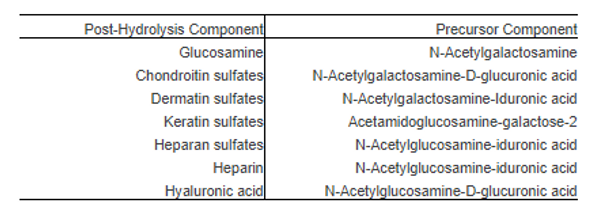
Bovine cartilage preparations have been used historically as wound healing agents and for chronic inflammatory conditions including osteoarthritis.59 Using enzymatic or chemical hydrolysis, cartilage can be disassembled to its lower molecular weight components which are demonstrably more bioavailable when administered orally. Depending on the method of hydrolysis, varying proportions of glucosamine and glycosaminoglycans are generated (Table 7). Oral consumption of cartilage or cartilage preparations are then further degraded through digestion and reabsorbed into the body for further metabolism and tissue distribution.
Table 7. Post-hydrolysis components of bovine cartilage.
Discussion & Conclusions
Glucosamine, along with other related chondroprotective compounds, has a long history of use for conditions of arthritis in humans, dogs, and horses. As early as the 1950’s, cartilage extracts containing glucosamine were injected into the arthritic joint. Beginning in the 1980’s, purified glucosamine salts were administered orally. Products today consist of either the sulfate or chloride salt of glucosamine as a stand-alone agent or in combination with chondroitin sulfate and other vitamins and minerals. There are over 15 clinical studies on the use and safety of glucosamine for osteoarthritis. These studies have mostly been conducted on humans using clinical evaluations, radiographs, and biochemical markers as endpoints.
A meta-analysis of glucosamine and chondroitin was published in the Journal of the American Medical Association in 2000. After correcting for bias and trial design, the authors concluded that some degree of efficacy appears probable for these substances. 60 The authors further state that even a modest degree of efficacy could have clinical utility given the safety of glucosamine and chondroitin.
Glucosamine is one of the compounds that comprise the hexosamine pathway (Fig. 2). Recent studies have supported the hypothesis that the hexosamine pathway serves as a “nutrient sensor” for the body and is part of the feedback loop for insulin-mediated glucose disposal rates (GDR) and leptin production. 61-66 It is well established in the rat model that perfusions of high levels of glucosamine can induce insulin insensitivity and mimic the symptoms of type-II diabetes. Further supporting this effect, recent work has demonstrated that over-expression of GFAT (enzyme responsible for producing glucosamine-6-phosphate, Fig 2) produced insulin resistance in mice. However, it is important to note that these effects have not been observed in humans or dogs or if these effects are relevant in rodents when administered clinically therapeutic doses.35,49
Natural sources of glucosamine are both historically, and currently, a component of canine and feline diets. These components are digested, absorbed, and metabolized into various nutrient building blocks suitable for incorporation into cartilage and surrounding matrix. As a process of aging, the endogenous production of glucosamine may decrease, shifting the equilibrium of cartilage remodeling to a catabolic state. Supplementing the diet with natural sources of glucosamine (processed from cartilage, chitin, etc.) would be a safe and effective method to chronically administer chondroprotective agents that nutritionally support articular joint metabolism contributing to the well-being of the dog or cat.
References:
1. Ettinger SJ, Feldman ED eds. Textbook of Veterinary Internal Medicine, Diseases of the Dog and Cat, 5th ed. New York, NY: W.B. Saunders Company; 2000; 2:1862-1863.
2. Johnston SA. Osteoarthritis: Joint Anatomy, Physiology, and Pathobiology. Vet. Clin. North Am. (Small Anim. Pract.) 1997;27:699-723.
3. McLaughlin RM, Roush JK, Medical therapy for patients with osteoarthritis. Veterinary Medicine, 2002;2:135-144.
4. Proprietary market research, 1996. Veterinary sample size: 200. Data on file, Pfizer Animal Health.
5. Logan EI, Wiggs RB, Zetner K, et al. Dental Disease. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, eds. Small Animal Clinical Nutrition. 4th Ed. Marceline, MO: Walsworth Publishing Company; 2000:479.
6. Kealy RD, Lawler DF, Ballam JM, et al. Effects of diet restriction on life span and age-related changes in dogs. JAVMA 2002;220:1315-1320.
7. Hardie EM, Management of osteoarthritis in cats. Veterinary Clinics of North America: Small Animal Practice, Osteoarthritis 1997;27:945-953.
8. Millis DL, Feline Joint Problems. Proceeding of the Fifth ACVS Veterinary Symposium,Chicago, 1995, p 533.
9. Bennett D, Osteoarthritis-its classification, pathogenesis and clinical relevance. Voor Diergeneeskunde 1993;118(suppl):19S.
10. Wolf A, Denoff D, Scherk-Nixon M, et al, Roundtable discussion: The geriatric cat. Feline Practice 1996 24:8 and 24:5.
11. Merck Veterinary Manual Online. http://www.merckvetmanual.com.
12. Alberts B, Bray D, Lewis J, et al. Molecular Biology of the Cell. New York, NY: GarlandPublishing; 1989;2:802-808.
13. Pavelká K, Gatterová J, Olejarová M, et al. Glucosamine sulfate use and delay of progression of knee osteoarthritis. Arch Intern Med 2002;162:2113-2123.
14. Reginster JY, Deroisy R, Rovati LC, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomized, placebo-controlled clinical trial. The Lancet, 2001;357:251-256.
15. Lippiello L, Woodward J, Karpman R, Hammad TA. In Vivo Chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clinical Orthopaedics and Related Research, 2000;381:229-240.
16. Houpt JB, McMillan R, Wein C, et al. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. The Journal of Rheumatology, 1999;26:2423-2430.
17. Noack W, Fischer M, Förster KK, et al. Glucosamine sulfate in osteoarthritis of the knee.Osteoarthritis and Cartilage 1994;2:51-59.
18. Müller-Faßbender H, Bach GL, Haase W, et al. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis and Cartilage 1994;2:61-69.
19. Tapadinhas MJ, Rivera IC, Bignamini AA. Oral glucosamine sulphate in the management of arthrosis: report on a multi-centre open investigation in Portugal. Pharmatherapeutica 1982;3:157-168.
20. Vaz AL. Double-blind clinical evaluation of the relative efficacy of ibuprofen and glucosamine sulphate in the management of osteoarthrosis of the knee in out-patients. Current Medical Research and Opinion 1982;8:145-149.
21. D’Ambrosio E, Casa B, Bompani R et al. Glucosamine sulphate: a controlled clinical investigation in arthrosis. Pharmatherapeutica 1981;2:504-508.
22. Crolle G, E’Este E. Glucosamine sulphate for the management of arthrosis: a controlled clinical investigation. Current Medical Research and Opinion 1980;7:104-109.
23. Adebowale A, Du Jianpin, Liang Z, et al. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharmaceutics & Drug Disposition 2002;23:217-225.
24. Setnikar I, Giacchetti, Zanolo G. Pharmacokinetics of glucosamine in the dog and man. Arzneim.-Forsch. Drug Res. 1986;36:729-735.
25. Setnikar I, Rovati LC, et al. Absorption, distribution, metabolism, and excretion of glucosamine sulfate. Arzneim-Forsch/Drug Res 2001;51:699-725.
26. Pujalte JM, Llavore EP, Ylescupidez FR. Double-blind clinical evaluation of oral glucosamine sulphate in the basic treatment of osteoarthrosis. Current Medical Research and Opinion 1980;7:111-114.
27. Setnikar I, Pacini MA, Revel L. Antiarthritic effects of glucosamine sulfate studied in animal models. Arzneim.-Forsch Drug Res 1991;41:542-545.
28. Setnikar I, Cereda R, Pacini MA, et al. Antireactive properties of glucosamine sulfate. Arzneim.-Forsch Drug Res 1991;41:157-161.
29. McNamara PS, Barr SC, Hollis N. Hematologic, hemostatic, and biochemical effects in dogsreceiving an oral chondroprotective agent for thirty days. AJVR 1996;57:1390-1394.
30. Rossetti L, Hawkins M, Chen W, et al. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J Clin Invest 1995;96:132-140.
31. Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating desensitization of the glucose transport system: role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 1992;266:4706-4712.
32. Marshall S, Bacote V, Traxinger RR. Complete inhibition of glucose-induced desensitization of the glucose transport system b inhibitors of mRNA synthesis. J Biol Chem 1991;266;10155-10161.
33. Marshall S, Garvey WT, Traxinger RR. New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. Fed Am Soc Exp Biol 1991;5:3031-3036.
34. Traxinger RR, Marchall S. Coordinate regulation of glutamine:fructose-6-P amidotransferase activity by insulin, glucose and glutamine. J Biol Chem 1991;266:10148-10154.
35. Pouwels M-JJ, Jacobs JR, Span PN, et al. Short-term glucosamine infusion does not affect insulin sensitivity in humans. J Clin Endocrinol Metab 2001;86:2099-2103.
36. Baron AD, Zhu JS, Zhu JH, et al. Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle: implications for glucose toxicity. J Clin Invest1995;96:2792-2801.
37. Cooksey RC, Hebert LF, Zhy JH, et al. Mechanism of hexosamine-induced insulin resistance in transgenic mice over-expressing glutamine:fructose-6-hosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology 1999;140:1151-1157.
38. Robinson KA, Sens DA, Buse MG. Pre-exposure to glucosamine induces insulin resistance of glucose transport and glycogen synthesis in isolated rate skeletal muscle: study of mechanisms in muscle and in rat-1 fibroblast over-expressing the human insulin receptor. Diabetes 1993;42:314-320.
39. Crook ED, Zhou J, Daniels M, et al. Regulation of glycogen synthase by glucose, glucosamine, and glutamine:fructose-6-phosphate amidotransferase. Diabetes 1995;44:314-320.
40. Crook ED, McClain DA. Regulation of glycogen synthase and protein phosphatase-1 by hexosamines. Diabetes 1996;45:322-327.
41. Hresko RC, Heimberg H, Chi MM, et al. Glucosamine-induced insulin resistance in 3T3-L1adipocytes is caused by depletion of intracellular ATP. J Biol Chem 1998;273:20658-20668.
42. Hawkins M, Barzilai N, Liu R, et al. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 1997;99:2173-2182.
43. McClain DA, Crook ED. Hexosamines and insulin resistance. Diabetes 1996;45:1003-1009.
44. Wang J, Liu R, Hawkins, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998;393:684-688.
45. Monauni T, Zenti MG, Cretti A, Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes 2000;49:926-935.
46. Giaccari A, Morviducci L, Zorretta D, et al. In vivo effects of glucosamine on insulin secretion and insulin sensitivity in the rat: possible relevance to the maladaptive responses to chronic hyperglycemia. Diabetologia 1995;38:518-524.
47. Balkan B, Dunning BE. Glucosamine inhibits glucokinase in vitro and produces a glucose-specific impairment of in vivo insulin secretion in rats. Diabetes 1994;43:1173-1179.
48. Shankar RR, Zhu JS, Baron AD. Glucosamine infusion in rats mimics the beta-cell dysfunction of non-insulin-dependent diabetes mellitus. Metabolism 1998;4:573-577.
49. Echard BW, Talpur NA, Funk KA, et al. Effects of oral glucosamine and chondroitin sulfate alone and in combination on the metabolism of SHR and SD rats. Mol Cell Biochem 2001;225:85-91.
50. Rodén L. Effect of hexosamines on the synthesis of chondroitin sulphuric acid in vitro. Ark.Kemi 1956;10:345-352.
51. Karzel K, Domenjoz R, et al. Effects of hexosamine derivatives and uronic acid derivatives on glycosaminoglycane metabolism of fibroblast cultures. Pharmacology 1971;5:337-345.
52. Bassleer C, Henrotin Y, Franchimont P. In-vitro evaluation of drugs proposed as chondroprotective agents. Int J Tiss Reac 1992;14:231-241.
53. Sandy JD, Gamett D, Thompson V, et al. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem J 1998;335:59-66.
54. Lippiello L, Idouraine A, McNamara PS, et al. Cartilage stimulatory and antiproteolytic activity is presenting sera of dogs treated with a chondroprotective agent. Canine Practice 1999;24:18-19.
55. Piperno M, Reboul P, Helio Le Graverand MP, et al. Glucosamine sulfate modulated dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthritis and Cartilage. 2000;8:207-212.
56. Shikhman AR, Kuhn K, Alaaeddine N, et al. N-Acetylglucosamine prevents IL-1b-mediated activation of human chondrocytes. The Journal of Immunology 2001;166:5155-5160.
57. Fenton JI, Chlebek-Brown KA, Caron JP, et al. Effect of glucosamine on interleukin-1-conditioned articular cartilage. Equine Vet J Suppl 2002;34:219-223.
58. Hua J, Sakamoto K, Nagaoka I. Inhibitory actions of glucosamine, a therapeutic agent for osteoarthritis, on the functions of neutrophils. Journal of Leukocyte Biology 2002;71:632-640.
59. Prudden JF, Balassa LL. The biological activity of bovine cartilage preparations. Seminars in Arthritis and Rheumatism 1974;3:287-321.
60. McAlindon TE, La Valley MP, Gulin JP, et al. Glucosamine and chondroitin for treatment of steoarthritis: a systematic quality assessment and meta-analysis. JAMA; 2000;283:1469-1475.
61. Daniels MC, Ciaraldi TP, Nikoulina S, et al. Glutamine:fructose-6-phosphate amidotransferase activity in cultured human skeletal muscle cells. The Journal of Clinical Investigation1996;97:1235-1241.
62. Wang J, Liu R, Hawkings M, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998;393:684-687.
63. Considine RV, Cooksey RC, Williams LB, et al. Hexosamines regulate leptin production in human subcutaneous adipocytes. The Journal of Clinical Endocrinology & Metabolism2000;85:3551-3556.
64. McClain DA, Alexander T, Cooksey RC, et al. Hexosamines stimulate leptin production in transgenic mice. Endocrinology 2000;141:1999-2002.
65. Emilsson V, O’Dowd J, Nolan AL, et al. Hexosamines and nutrient excess induce leptin production and leptin receptor activation in pancreatic islets and clonal b-cells. Endocrinology2001;142:4414-4419.
66. Zhang P, Klenk ES, Lazzaro MA, et al. Hexosamines regulate leptin production in 3T3-L1adipocytes through transcriptional mechanisms. Endocrinology 2002;143:99-106.







מאת: ד"ר שלומי עמיאל וד"ר רואי להב המרפאה הווטרינרית בשבי ציון
© כל הזכויות שמורות לכותבי המאמר: ד"ר זרצקי את עמיאל


